


 WHO WE ARE
WHO WE ARE
 WHAT WE DO
WHAT WE DO
 PUBLICATIONS
PUBLICATIONS
Tissue-Grown Corporation was founded in 1986 and has an established business in the propagation of disease-indexed horticultural crops.
Tissue-Grown is a new type of technology company, combining commercial micropropagation and greenhouse production expertise with the disciplines of cell genetics and cell physiology.


The core business focuses on the mass production of fruit and nut tree rootstocks including , , , and cherries.
Tissue-Grown Corporation uses best practice methods and research and development investments to be a forward thinking green company. Due to these guidelines Tissue-Grown Corp. has grown rapidly and is actively seeking expansion, investments, commercial research partners, and new high volume projects.
Carolyn J. Sluis
is an industry leader in plant tissue culture and has been working in the field of plant micropropagation and potato tissue culture since 1975. She is listed as inventor on five patent applications (issued and pending) regarding inventions of new tissue culture techniques. Ms. Sluis is the principal founder and Chief Executive Officer of Tissue-Grown Corporation.
Ms. Sluis' formal training comes from education in plant environmental biology, at University of California Santa Barbara and San Diego campuses. She also completed course work with the "father of commercial plant tissue culture" Dr. Toshio Murashige, at the University of California, Riverside. From 1975-1977, Ms. Sluis was employed as the tissue culture manager for K.M. Nurseries in California, one of the first commercial tissue culture laboratories. In 1978, Ms. Sluis started a tissue culture laboratory for NPI (then Native Plants, Inc.) a biotechnology company based in Salt Lake City, Utah. Her research there resulted in development of production systems for a number of endangered species and unusual native species. She initiated the potato project at NPI, which produced the first commercial virus tested potato plantlets in the United States. From 1981-1986 Ms. Sluis was a Research Scientist and Research Group Leader at Plant Genetics, Inc. (now a division of Calgene, Inc.). She led the group responsible for the development of one of Plant Genetics, Inc.'s key products: potato minitubers. In addition to research, she pioneered commercial potato minituber production, now an industry standard for the production of elite seed potatoes. Ms. Sluis has consulted internationally with companies in Thailand, Japan, France, Australia, Germany and the Netherlands. She is a key consultant for Floratiss, B.V. in the Netherlands, which was one of the world's largest lily bulb micropropagation laboratories in Portugal and South Africa.
Travis Johansen has worked with Tissue-Grown since 1999. Mr. Johansen is a graduate of Hawai'i Pacific University, earning a Bachelor's Degree of Science in Computer Science in 2010, and has studied at Santa Barbara City College and the University of California, Santa Barbara. He currently designs, implements, and maintains software and information systems; oversees production models for delivery goals; and handles administrative tasks at Tissue-Grown.
Brett Gytri is a graduate of UCSB with a degree in Pharmacology, and is a researcher at Tissue-Grown Corporation. Mr. Gytri has worked on and had success with research at Tissue-Grown including increasing the rooting rate for pistachio rootstock, increasing the efficiency of plant production in the lab, developing new media through Design of Experiment, and testing proprietary methods.
Tissue-Grown Corporation employs nearly 100 people and operates a 9,300 square foot laboratory designed around 3 primary tissue culture production subdivisions, in Santa Paula, California. The laboratory encompasses 3000 sq.ft. of media preparation, dishwashing, autoclaving and storage areas.
The conventional micropropagation and the photoautotrophic miniplug production divisions each have 2400 sq.ft. of transfer hoods and large climate-controlled rooms with rolling culture shelves. Each culture room can hold over 1 million plantlets at any point in time, and for the past several years the lab has operated with at least 1 million tree cultures in inventory at any given time.
There are several research areas for designated projects in photoautotrophy, with controlled CO2 rooms, innovative lighting research for photomorphogenesis effects, and chopper-lighting (www.chopperlight.de) for light color control and energy savings.
On site, Tissue-Grown also has refrigerated coolers, research and propagation greenhouses, and warehouse space. The greenhouses contain close to 20,000 square feet of bench space, use extensive proprietary technology, and are managed by state of the art environmental controls.
Tissue-Grown Corporation is located in beautiful Santa Paula, California.
Santa Paula is in Southern California and is known as the Citrus Capitol of the World because of its lush top soil and Mediterranean climate.
Shipping, both nationally and internationally, is possible via Los Angeles International Airport, just one hour away. Tissue-Grown is very experienced in the management of international shipments - such as working with import permits, phytosanitary certificates, commercial invoices, and customs brokers.

The coffee trees produced in liners at Tissue-Grown are 100% started from tissue culture.
Not only does this allow us to meet a high volume demand for orchard replacement or planting, but it also produces a tree which is vigoursly growing and free from disease or virus.

, or send an email to sales at tissuegrown.com, to discuss how these new varieties or services can benefit your farm or nursery.

Our coffee protocol was developed to help meet the demands that farmers face when planting a crop which must be both resistant to Coffee Leaf Rust and at the same time provide the highest quality cupping available.
Tissue-Grown has researched and developed a robust in-vitro model for the production of coffee in tissue culture. The genesis of this work was in response to the current threat of coffee leaf rust (caused by Hemileia vastatrix), which our varieties are resistant to.


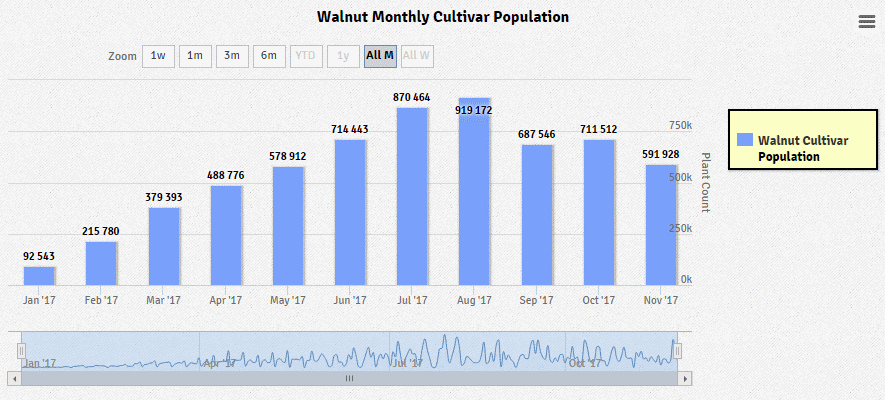
Tissue-Grown Corporation offers state-of-the-art clonal in vitro propagation services for walnut rootstock.
Tissue-Grown Corporation has proven the ability to mass produce Walnuts as well, with the capacity to produce and ship both locally and internationally.
In addition to having completed the shipment of hundreds of thousands of walnuts, Tissue-Grown Corporation is also actively researching the ability to graft this crop prior to the field.
Many plant varieties are difficult to propagate, but have been researched and proven to be superior selections. Tissue-Grown Corporation offers state-of-the-art clonal in vitro mass propagation services for hard to propagate crops and varieties.
With a strong history of developing well researched production systems for difficult crops, we can help you with your difficult variety.
to discuss the possibility of getting started on mass producing your difficult crop.
 Pages 231-251 |
Integrating Automation Technologies With Commercial Micropropagation An economic perspective Written By: Carolyn J. Sluis
Sluis, C.J. 2005. Integrating Automation Technologies With Commercial Micropropagation.
IN: Plant Tissue Culture Engineering |
Abstract
Significant advances have been made in micropropagation technologies at both the laboratory and greenhouse levels which offer promise for future mechanization. Several mechanized or automated processes are already seen in commercial practice. This chapter will discuss the various types of mechanization with an emphasis on commercial applications throughout the world. Sections will be devoted to the individual technologies followed by specific analysis of the advantages and disadvantages of the systems relative to commercial implementation. Batch fermentation coupled with bud cluster growth habit modification, and robotics, guided by vision systems, in both laboratory and greenhouse applications will be reviewed. Significant factors key to the commercialization of automation will be discussed, including: 1) bacterial contamination management, starting with culture indexing; 2) economics, especially as regards seasonal production and competitive labor; and, 3) growth habit modification strategies, focussing on bud clusters versus linear growth habits. Potato tissue culture illustrates the complexity of the economic and biological issues, internationally, and will be discussed in depth as an example of a species which can be micropropagated either via linear growth habit, suitable for robotic manipulations, or as a bud clusters, suitable for indiscriminate chopping.
Keywords: micropropagation, biofermentation, bioreactors, cost accounting, robotics
Originally presented at the 2015 In Vitro Biology Meeting of the Society for In Vitro Biology
Presented by Brett Gytri. Co-authored by Carolyn Sluis.
Researchers in universities across the globe have made great strides toward understanding many of the fundamental underlying principals of plant growth regulation, using the model plant Arabidopsis.
This has resulted in a wealth of avenues to explore for better control of plant development and morphology during propagation in vitro. The challenge in the current economic marketplace is to create reliable, consistent, high volume plant production systems using the best research tools and the best scientific breakthroughs, while keeping in mind both the economics and the marketability of the end product.
Experimental design and analysis programs have become increasingly accessible to the tissue culture researcher and can help create multifactorial experimental designs which allow evaluation of many interacting factors in a relatively short time frame simultaneously.
The steps comprising a tissue culture propagation system can be modeled to produce the maximum number of plants while factoring in the best use of labor and materials. The physical parameters of the culture system: the vessels, gas environment and substrate, play a large role in plant quality. Liquid based systems can be compatible with mechanization. Photoautotrophic systems can improve plant quality and normalcy.
Blending the many options into a functional economic propagation system can be challenging. However, most commercial plant tissue culture laboratories subculture their plants in small vessels with gelled, sugar-containing media. Why? We will explore the reasons for this and examine the research tools available for attaining the goal of high volume micropropagation of economically viable crops in novel and sometimes unexpected ways.
Principals Of Germplasm And Disease-Indexed Line Maintenance
Once a line has been established as meeting the standards for germplasm it can be maintained for many years if proper care is given to details. The most common mistakes in germplasm maintenance involve line identity, line purity and secondary testing.

Each germplasm line should have a double identity system: both an initial
source number or code and a name or key identifier. Lines should be
maintained in culture vessels which are contained in rigid blocks in
the germplasm culture area, such as in plastic trays or racks. Each
germplasm block is labeled with all relevant identification: accession
number, name or breeding number, description, date of accession, etc.
Accession numbers should refer to a log kept with additional relevant
information (disease testing status, source or origin of the line,
etc.). Each test tube or culture vessel within the block is labeled
with the accession number.
Operators who are working with the
germplasm do not work with more than one line in the workstation at any
one time. Only a portion of the culture base is subcultured at any one
time, so that a back up of older cultures is always maintained
alongside the newly subcultured test tubes.
No germplasm lines are ever derived from left-over propagation runs. Germplasm is a one-way culture base; cultures which exit germplasm can never come back in. They can go out to testing or out to production, but cultures in germplasm must not be contaminated by cultures from other sources. Germplasm should be maintained under minimal conditions, such as growth regulator free medium, to ensure the least amount of genetic change. Ideally, germplasm is maintained in a culture room or area separated from the production culture base. Also, a separate area for new lines and quarantined (untested) lines should be enforced.
Annual certification of germplasm linesEvery year a single test tube is divided into 3-4 new test tubes, 3 for testing of key viruses, bacteria, etc. and one for starting the next year's germplasm. After 6-8 weeks, when viability and test results have been confirmed, the remaining back-up test tubes in germplasm are destroyed. Annual grow-out for verification of line identity and genetic purity is also sometimes warranted. Slight changes in genetics have been well documented, especially in certain species.
Propagation or production increases starting with germplasm linesFor production a single germplasm test tube should be selected for increase. This test tube is divided and increased to meet production needs. A single test tube of this individual is kept back, either for testing or grow-out to verify the line characteristics.
Maintenance of Virus-Elite Tissue Cultures
A clean culture line can, in theory, become infected or reinfected
while in tissue culture. We have never experienced this directly, but
have seen it on rare occasion, in our consultations. Here are some
possible scenarios:
Infection via insects
Infestations of insects in test tube cultures does occur. The most common insects
which are capable of thriving in tissue cultures are thrips and mites.
Thrips are well known carriers of viruses. Thrip infestations are most
hazardous if the original insect goes unnoticed and a population
sterile offspring is developed. Sterile thrips can be easily
subcultured along with the plantlets, as they are often very tiny and
not noticeable. Still, thrips need a source of viruses in order to
spread them. So the question of greenhouse or outside source of the
viruses in question should always be addressed at the same time.
Infection via mechanical transfer
If virus-infected cultures co-exist in the laboratory with the virus
indexed cultures, then it is possible to cross contaminate by operator
error, typically from poor sterilization of tools between each line.
Reinitiation of lines from greenhouse plants or propagation cultures
If a germplasm line has been lost to some catastrophe, such as medium
contamination, etc., then it is possible that the line was reinitiated
into tissue culture from an infected source, such as a greenhouse
plant, and insufficient testing was done to ascertain the virus status
of the parent line and its tissue culture derivatives.
"Infection" via mislabeling or line identity errors
A line might appear to be re-infected when in fact it is a mislabeled
line. The line in question should probably be grown out and verified as
the actual plant it is supposed to be.
"Infection" via testing errors
Always question initial results. Testing labs are not infallible. Always make
sure the tests have been repeated as blinds (coded labels) before
accepting a result. Always examine the optical density readings for the
positive and negative controls for indications of testing accuracy.
ELISA based testing is rated on statistical significance in the
difference between the positive and negative controls and the sample
result. It is sometimes not a black and white answer. Some viruses are
more problematic in testing than others.
Infection because the line was not perfectly virus-free originally
There are many different viruses and additional viruses may become relevant
for testing which were not originally part of the elite line
requirements. It is always important to check the prior test results
for the viruses in question. Also, it is possible to have high titer
negatives initially which rise over time to become positives. This is
usually only the case over the first 6 months of a line in tissue
culture.
Ideally, lines are tested using the broadest based
testing system, such as electron microscopy or POTY virus tests, which
can reduce the potential for non-obvious viruses present in the culture
base.